Today we started our unit on Triboluminescence. Triboluminescence is the formation of light caused by mechanical action, such as rubbing, breaking, or crushing. There are many different materials that exhibit this type of behavior: quartz, scotch tape, computer labels, duct tape, and Wint-O-Green LifeSavers.
When, for example a LifeSavers candy is broken, chemical bonds in the candy break resulting in an unequal distribution of charge on the pieces. This creates a large voltage difference between the pieces. As a result of this difference, electricity flows, symbolized by the lightning bolts, between the pieces in the diagram below. The electricity excites the electrons in the nitrogen molecules in the air, the purple spheres, surrounding the candy. Air is approximately 78% nitrogen. The nitrogen molecules emit ultraviolet light, light purple squiggles, which is absorbed by electrons in the oil of wintergreen molecules in the LifeSaver candy. The molecules lose some energy to vibration before the electrons relax back down to their ground state. Because some of the energy is lost to vibration, when the electrons relax back down to their ground state, the energy that is emitted is lower than the energy that excited the molecule and the wavelength is shifted from the UV portion of the spectrum, which we cannot see, into the visible portion of the spectrum in the form of blue light, which is the spark we see.
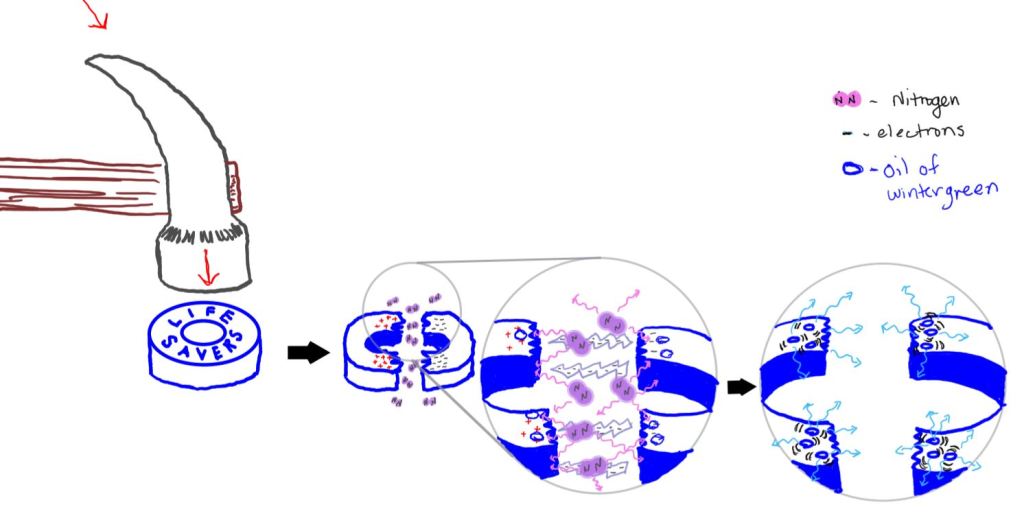
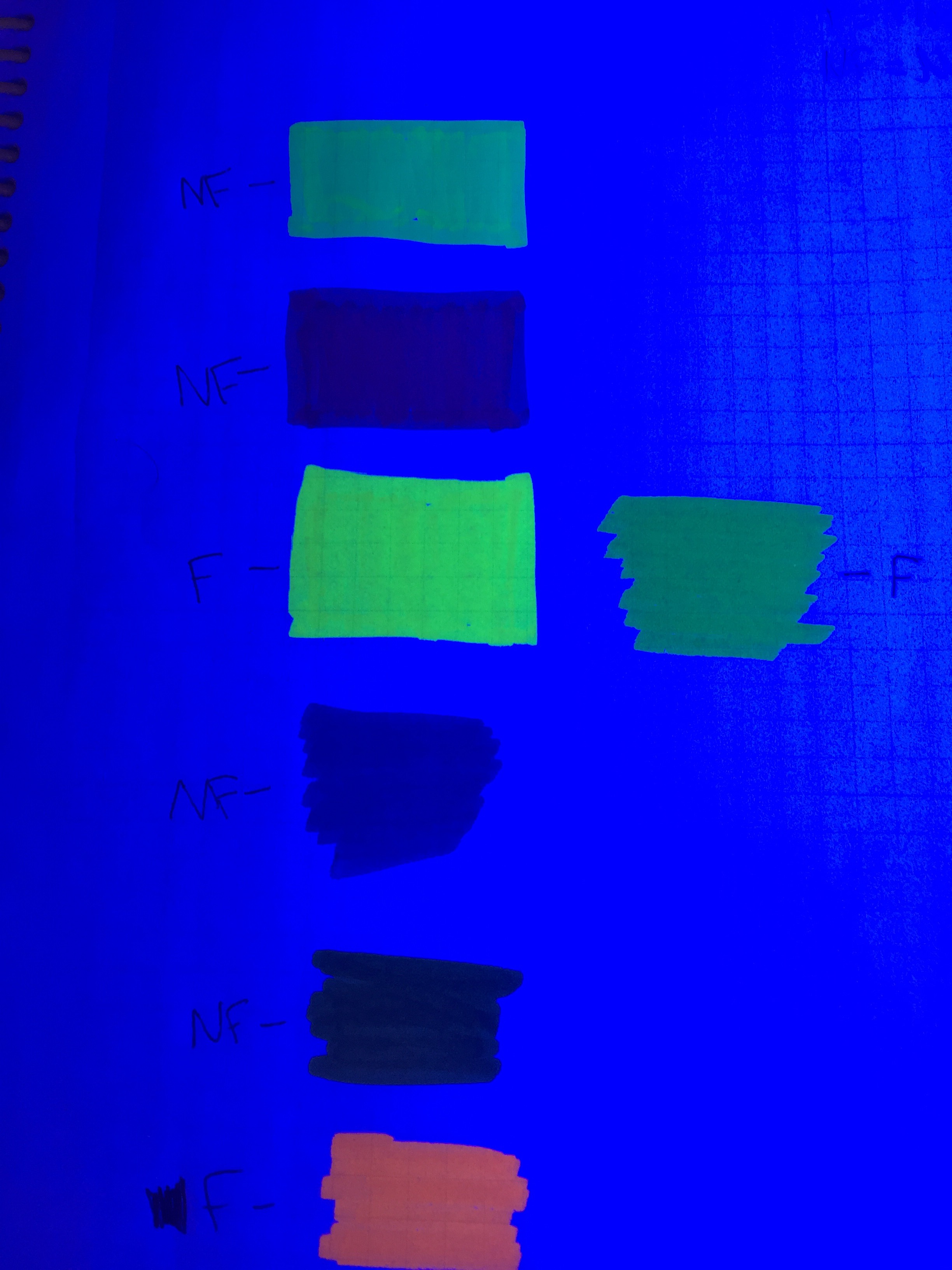
In other words, the triboluminscence causes the oil of wintergreen in the candy to fluoresce. Fluorescence occurs when electrons in a molecule absorb energy with a shorter wavelength and then re-emit it at a lower energy longer wavelength. In commercial products, fluorescent dyes are used to make objects appear brighter than their surroundings. The dyes absorb UV radiation which we cannot see, lose some of the energy to vibration and re-emit the light in the visible portion of the spectrum. The figure below shows different dyes under room light and fluorescing under UV light.


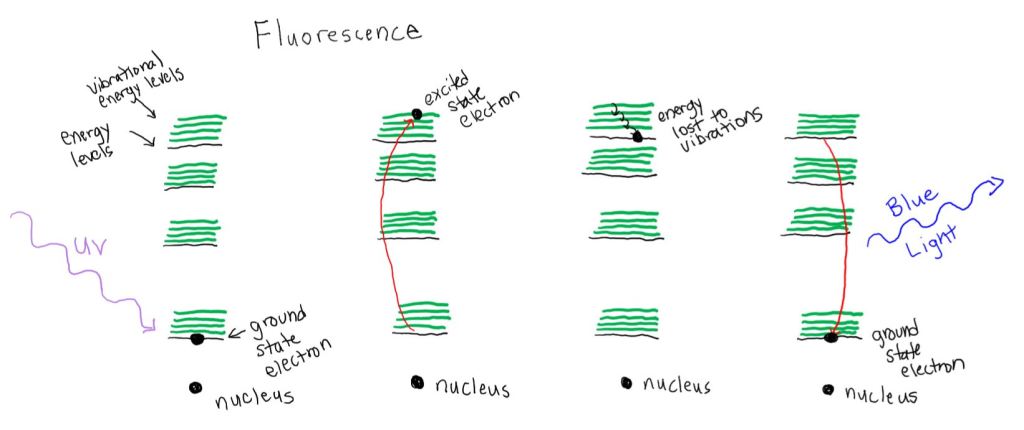
Ultraviolet light having an energy equal to the difference between the first and the fourth energy levels is absorbed by the electron. The electron is promoted to the fourth energy level, this is a high energy state or excited state. The electron loses some energy to vibration. When the electron relaxes back down to its ground state, it has less energy than it absorbed. So the light that is emitted has a longer wavelength, and is now in the visible portion of the spectrum, which we can see. This is an over simplification but conveys the general idea of what is happening.
We introduced the concept of triboluminescence by having the students observe this process in a variety of different materials such as alluvial quartz, computer labels, scotch tape, LifeSavers candy, and duct tape. We posed a question to our students: can you capture the triboluminescene using your cell phone? The students experimented for an entire period before realizing that it was very difficult to capture the light created by this process.

Next, the students investigated different highlighter markers to determine if they contained fluorescent dyes. Then, they used the fluorescent highlighters on the lifesavers to see if they could shift the color that the lifesaver produced under UV light.
Next week, the students will try to capture the spark and shift the color on film.






Leave a comment